do i need to autoclave 1m naoh freshly prepared solution|can naoh be autoclaved : chain store Add 0.2 ml DEPC to 100 ml of the solution to be treated. Shake vigorously to dissolve the DEPC. Autoclave the solution to inactivate the remaining DEPC. CAUTION: Wear gloves and use a fume hood when using DEPC, as it is a suspected carcinogen. Many investigators keep the solutions they use for RNA work separate to ensure that fidirtyfl Tests were performed measuring the temperature, ionic conductivity, and compaction in 16 to 200 ply thick graphite-epoxy laminates made of either Fiberite T300/976 (tape or fabric) or .Autoclave molding is a closed-molding manufacturing process. It entails curing composite materials inside a mold or bag. Once the composites are placed inside the mold, it is deoxygenated, which produces an equal distribution of .
{plog:ftitle_list}
autoclave vertical analÓgica, capacidade 75 litros, com tampa em aÇo inox, cÂmara interna medindo 40 cm diÂmetro x 60 cm altura, equipada com manÔmetro e 2 cestos, 220v - .
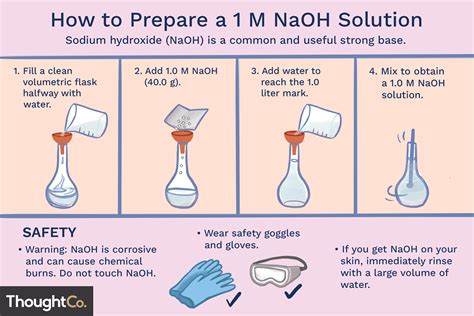
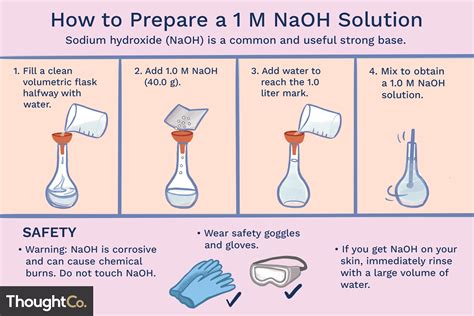
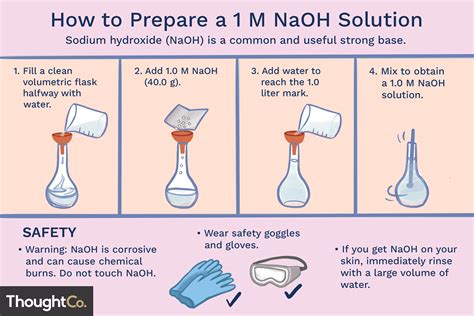
I need to prepare 100ml 1N NaOH solution. I dissolved 8 g of NaOH in 100ml of sterilised distilled water. I use this solution to extract the melanin content from the cells by incubating at 80 degree celsius for 1 hr. Do I still need to autoclave the solutions before I use it .
lamination without autoclave
NaOH is a strong alkaline, they shouldn't be any growth inside the solution. Thanks. I prepar. Special care is required to prepare a solution of sodium hydroxide or NaOH in water because considerable heat is liberated by the exothermic .Add 0.2 ml DEPC to 100 ml of the solution to be treated. Shake vigorously to dissolve the DEPC. Autoclave the solution to inactivate the remaining DEPC. CAUTION: Wear gloves and use a fume hood when using DEPC, as it is a suspected carcinogen. Many investigators keep the solutions they use for RNA work separate to ensure that fidirtyfl
preparation of naoh solution
This means that the normality of a 10M solution of NaOH is equal to 10N. The molecular weight of NaOH is 40. This means you need to dissolve 40 g of NaOH in water to obtain a 1 liter of 1M (or 1N) NaOH solution. To prepare a 10M . A 100 ml $\ce{HCl}$ solution has a pH of .7$. You want the solution to be of pH 4.5. You have a solution of \ \mathrm M$ $\ce{NaOH}$. How much $\ce{NaOH}$ do you need to add to to the 0\ \m.
For example, a 70 % (v/v) solution of ethanol can be prepared by dissolving 70 mL of 100% (i.e., 200 proof) ethanol in a total solution volume of 100 mL. Other factors may also be important when deciding on the type of percent solution to prepare. For example, if the percent solution under consideration is to be used at widely different . To prepare 1M Tris-HCl from a 10mM solution, you would need to dilute the 10mM solution by a factor of 100. This means you would mix 1 part of the 10mM solution with 99 parts of water to achieve a .To prepare a solution of specific molarity based on mass, please use the Mass Molarity Calculator. To dilute a solution of concentrated acid or base of known w/w% strength, please use the Acid & Base Molarity Calculator. Stock concentration: Desired final volume: Desired concentration: Find your pH balance with our .A 1M DTT solution can be prepared by dissolving 3.86 g of DTT in water to a final volume of 25 ml. REQUIREMENTS. Reagents and solutions DL-Dithiothreitol (C 4 H 10 O 2 S 2) . Do not autoclave the solution. Autoclaving will destroy the DTT. Storage DTT solution should be stored at .
how to make naoh solutions
how to make naoh
Step 3: Sterilize the solution by filtering through a sterile filter unit containing a membrane with porosity 0.22 µm or less. Precaution: We don’t recommend autoclaving the solution. Note: Freshly prepared solution will appear clear colourless liquid which can turn into yellowish with time. Storage Stored the solution at 4° C in a brown bottle
I suggest that you verify the pH of the freshly prepared MnCl 2 sol. in water ― it . I need to dissolve it in water for the preparation of SEM-TB media. I prepared 1M stock solution but it is .
Titrate solution to desired pH using NaOH pellets. The expected amount of NaOH to use for a pH of 8.0 can be found in the table above. Once completely dissolved, transfer the solution to a sterile volumetric flask and adjust the final volume using dH 2 O , then return it to the container.
If you use solid NaOH pellets, you’ll need 18 to 20 grams of NaOH. Add the last of the NaOH slowly so that you don’t overshoot the pH. You may wish to switch from solid NaOH to a solution toward the end for more precise control. The EDTA will slowly go into solution as the pH nears 8.0. Dilute the solution to 1 L with distilled water.To prepare 1000 mL of a 0.1 mol/L solution of AlCl3 we have to dissolve 24.1433 g of AlCl3×6H2O (96 % purity) in deionized or distilled water. After the solid is completely dissolved, dilute the solution to a final volume with deionized (distilled) water. we will need to dilute 13.72 mL of 73 % AlCl3×6H2O to a final volume with deionized . To prepare 500 mL of 1M solution, you would need to take (1/6)th of the volume of the 6M solution, which is (1/6) x 500 mL = 83.33 mL of the 6M solution. Dilute this with water to reach a final .Note: Do not autoclave DTT or solutions containing it. 10 mg/ml DNA (Salmon Sperm) Sonicated, denatured salmon sperm DNA is commercially available at a con-centration of 10 mg/ml but is fairly expensive. A large economical supply of salmon sperm DNA stock solution can be prepared in the laboratory, although the process is lengthy. PB GA MC2 C
2H 2 O, in enough ethanol to make exactly 500 mL of solution. What is the molar concentration of CoCl 2 Preparing Stock Solutions. A stock solution is prepared by weighing out an appropriate portion of a pure solid or by measuring out an appropriate volume of a pure liquid, placing it in a suitable flask, and diluting to a known volume. Exactly how one measure’s the reagent depends on the desired concentration unit. For example, to prepare a solution with a .
This molarity calculator is a tool for converting the mass concentration of any solution to molar concentration (or recalculating grams per ml to moles). You can also calculate the mass of a substance needed to achieve a desired molarity. This article will provide you with the molarity definition and the molarity formula.. To understand the topic as a whole, you will want to learn .The first thing you will need to do in lab this week is to prepare a -0.1M solution of NaOH, which you will standardize. You will prepare 250-ml of this solution using a 30% (m/v) NaOH stock solution. How many mL of the NaOH stock solution will you need to .
Since ascorbic acid is highly unstable, you will have to prepare a fresh solution in water each time you perform your experiment unless and until you are using the stable form of ascorbic acid.Stir the mixture at 60˚C in ventilation hood (DO NOT Boil). PFA powder does not dissolve instantly, you need to raise the pH of the mixture by adding 5N NaOH drop by drop until a clear solution is formed. There may be small undissolved particles. Cool the solution to room temperature and filter to remove particles. Adjust the volume to 1L with . I need to prepare 100ml 1N NaOH solution. I dissolved 8 g of NaOH in 100ml of sterilised distilled water. I use this solution to extract the melanin content from the cells by incubating at 80 degree celsius for 1 hr. Do I still need to autoclave the solutions before I use it for my experiment?
To prepare 1000 mL of a 0.1 mol/L solution of AlCl3 we have to dissolve 24.1433 g of AlCl3×6H2O (96 % purity) in deionized or distilled water. After the solid is completely dissolved, dilute the solution to a final volume with deionized (distilled) water. we will need to dilute 13.72 mL of 73 % AlCl3×6H2O to a final volume with deionized .
how to dilute naoh
Procedure for Preparing 1N NaOH Solution. Step 1: Using the digital balance weigh accurately 40g of sodium hydroxide (NaOH). Step 2: Add precisely weighed NaOH to 500ml of distilled water in the volumetric flask. Step 3: Using the stirrer dissolve NaOH is the water. Step 4: Once the sodium hydroxide is dissolved let it cool to ambient temperature.Its a very routine process in our lab. We never purchase the solution. You need to know the molarity of the solution before going into the details. Lets consider it 1M, pH 9 and you need to .A detailed study of 7 freshly prepared culture media with the help of decimal dilutions of 21 pure cultures of bacteria, 10 anaerobic, shows that Trypticase Soy Broth (BBL) appears to be the best .To adjust your pH-meter, you must use the 3 pH standard solutions (pH4, 7 and 11) not only the pH4 standard. If your pH-meter indicate pH 2.0 with your pH4 standard, you have to adjust the pH to 4 .
The molecular mass of zinc acetate dihydrate is 219.5. Thus to prepare a 0.02 M solution in DI water requires 219.5*0.02 = 4.39 g/L. In the article 50 mL is mentioned as the quantity.
can you autoclave 1n naoh
To prevent burns, allow unit to cool before cleaning. Wipe with a soft dry cloth and occasionally with a damp cloth and mild soap or detergent. Drain water from reservoir using drain tube .
do i need to autoclave 1m naoh freshly prepared solution|can naoh be autoclaved